The following is a detailed description of the POP-OUT Trial which would be primarily of interest to researchers in clinical obstetrics or midwifery. Please note that our information sheet and more general information on the POP-OUT Trial are also available on this website. If you are keen on reading as much information as possible then please read on...
Protocol for the POP-OUT Trial
Title:
The POP-OUT Trial protocol: Persistent Occiput Posterior Position: OUTcomes following manual rotation
Scientific Title:
Among women at least 37 weeks' gestation at full cervical dilatation in labour with a fetus in the occiput posterior position, does attempted manual rotation to the occiput anterior position compared with a sham procedure reduce the chances of an operative delivery?
Registration:
Registered with the Australian New Zealand Clinical Trials Registry on 23rd September 2009 (ACTRN12609000833268). The power calculation was n = 254. The intention was that the 1st 30 participants would be analysed as a pilot study and that the main trial would continue using the same protocol.
Pilot study results showed that additional funding would be required and led to a change in the secondary outcomes. New trial commenced using the same power calculation. New secondary outcomes were pre-specified on successful peer reviewed NHMRC application for funding in March 2011.
First recruitment 19th April 2012.
As unable to modify the protocol, the trial was resubmitted for registration on 5th May 2012 (ACTRN12612001312831) (recruitment at this time 4/254 = 1.6%).
Due to technical difficulties with formatting separate components of combined secondary outcomes, registration not finalized until 19/12/2012 (recruitment at this time 35/254 = 14%).
Primary outcome unchanged since first registration September 2009.
Secondary outcomes unchanged since NHMRC grant application March 2011.
UTN: U1111-1127-4753
All items from the World Health Organization Trial Registration Data Set are included in this protocol.
Contacts:
Contacts for both public and scientific queries are Ms Hala Phipps/Dr Brad de Vries via.
Postal address:
C/- Women and Babies' Executive Unit
Level 5, Royal Prince Alfred Hospital
Missenden Rd
Camperdown, NSW, 2050
EMail:
bradley.devries@sswahs.nsw.gov.au
hala.phipps@sswahs.nsw.gov.au
Phone: 61-2-9515-8416
Website: Click on 'contacts' tab via www.popout.me
Countries of recruitment: Australia only
Health condition studied: occiput posterior position in the second stage of labour
Intervention studied: prophylactic manual rotation
Comparator: a 'sham' manual rotation
Protocol version 2 20/01/2012
Principle investigators:
Hala Phipps 1,2,3
PhD Candidate/Research Midwife
Tel: +61 2 95156079
Email: hala.phipps@email.cs.nsw.gov.au
Clinical Professor Jon A Hyett 1,2
Joint Head of the Discipline of Obstetrics, Gynaecology and Neonatology
Dr Sabrina Kuah 4
Director-Delivery Suite obstetrician & Senior Consultant O&G
Dr John Pardey 5
Visiting Medical Officer, Director of Obstetrics and Gynaecology
Dr Joanne Ludlow1
Senior staff specialist obstetrician
Associate Professor Andrew Bisits 6
Senior staff specialist obstetrician, Director of Birth Services
Dr Felicity Park 7
Staff specialist obstetrician
Dr David Kowalski 8
Visiting medical officer and specialist obstetrician
Dr Bradley de Vries 1,2
Staff Specialist Obstetrician
Investigators affiliation and protocol contribution
RPA Women & Babies, Royal Prince Alfred Hospital, Sydney, NSW, Australia
Discipline of Obstetrics, Gynaecology and Neonatology, University of Sydney, NSW, Australia
Faculty of Nursing and Midwifery, University of Sydney, NSW, Australia
Women's and Children's Hospital, Adelaide, SA, Australia
Nepean Hospital, Penrith, NSW, Australia
Royal Hospital for Women, Sydney, NSW, Australia
The John Hunter Hospital, Newcastle, NSW, Australia
Canterbury Hospital, Sydney, NSW, Australia
Trial sponsors:
Primary Sponsor: The University of Sydney
Secondary Sponsor: The Royal Prince Alfred Hospital, Sydney
The trial sponsor will have no role in collection, management, analysis, and interpretation of data; writing of the report; or the decision to submit the report for publication, and will have no authority over any of these activities.
Roles and responsibilities
Trial management Committee
The committee consists of Hala Phipps , Jon Hyett and Bradley de Vries who are responsible for the following:
Study planning
Organisation of Steering Committee meetings
Randomisation
Providing annual risk reports to the Ethics Committee and the Data Monitoring Committee
Reporting of any serious adverse events to the Data Monitoring Committee
Budget administration and organising contracts with individual centres
Providing advice for site investigators
Auditing and visiting sites
Data verification
Following up of study participants
Site investigators
In each participating centre a lead investigator (obstetrician) will be responsible for identification, recruitment data collection and completion of relevant trial forms, along with adherence with study protocol. Each lead investigator will be a steering committee member.
Steering Committee
The Steering Committee, will be chaired by BdV and all lead investigators will be steering committee members and are responsible for the following:
Recruitment of pregnant women on the study and liaising with principal investigators HP, JH and BD.
Reviewing progress of study and facilitating the smooth running of the trial.
Reporting the results of the trial
Data manager
Maintenance of trial IT system and data entry
Data verification
Research question
For women who are at least 37 weeks gestation whose baby is in the OP position early in the second stage of labour, does manual rotation compared with a “sham” rotation result in a reduction in operative delivery?
Background and rationale
Persistent occiput posterior (OP) position is associated with 18% of intrapartum caesarean sections and a high risk of assisted vaginal delivery 1-3. Caesarean section is now a major contributing factor to maternal mortality and morbidity following childbirth in developed countries 4;5. Obstetric intervention by forceps and ventouse delivery is associated with complications to the maternal genital tract and neonate respectively 6-8.
Manual rotation from the OP to the occiput anterior (OA) position is a safe, relatively simple and easy to perform procedure which could reduce the operative delivery rate (defined as vacuum delivery, forceps delivery and/or caesarean section) and therefore increase the chances of a normal vaginal birth 9. It is performed by only a minority of obstetricians and midwives in Australia and New Zealand yet is considered to be acceptable by the vast majority 10;11. However, obstetricians and midwives would perform a manual rotation if there was evidence that it reduced the risk of operative delivery to 50% or less 10;11 suggesting that demonstration of efficacy will translate into clinical practice.
Preliminary studies of efficacy are promising, but there has been no adequately powered randomized controlled trial (RCT) 12;13. It has been recommended that RCTs be conducted to explore the efficacy of manual rotation in the management of OP labours 14.
Epidemiology: The prevalence of the OP position is 15-32% at the onset of labour 15-18, 10-20% early in the second stage of labour and 5-8% at delivery 2;17;19;20. The operative delivery rate varies from 54% to 82% when OP position is present at delivery, compared with 6% to 22% when the fetus is in the more common OA position 3;16;19;20. When the OP position is present at the beginning of the second stage of labour, the operative delivery rate was about 70% in two higher risk cohorts 2;3.
Thus, of all women who plan to have a normal vaginal birth, 10-20% will have a fetus in the OP position early in the second stage of labour. These women will be eligible to have a manual rotation to modify their background risk of up to 70% of obstetric intervention with forceps, vacuum or caesarean section.
Complications of OP position: The OP position is associated with more frequent induction and augmentation of labour and prolonged first and second stage of 3;17;18;21, chorioamnionitis, post-partum haemorrhage, third and fourth degree perineal tears, wound infection and endometritis 22;23;23. Associated adverse neonatal outcomes include birth trauma, low 5 minute Apgar score, and admission to the neonatal intensive care unit 24.
The intervention in current practice
Manual rotation is a well-accepted component of obstetric practice, particularly in the context of rotating the fetus to the OA position immediately prior to the application of non-rotational forceps such as Neville-Barnes 25. However, it is also used commonly in a prophylactic setting (without assisted delivery) to reduce the complications associated with OP delivery 12;13. In a survey of obstetricians in Australia and New Zealand, 70% believed it was acceptable in a prophylactic setting; but only 38% had performed a manual rotation in the last year, and most of these had only performed one or two 10. Both obstetricians and midwives reported they would perform a manual rotation if there was evidence that it would reduce the chances of operative delivery from 68% to 50% or less 10;11. Thus demonstration of efficacy would provide substantial scope for the intervention to be introduced into widespread practice.
The efficacy of the intervention
Preliminary cohort studies report that manual rotation is associated with a reduction in caesarean section and adverse maternal outcomes:
In a retrospective cohort study, Schaffer et al (2006) (n=731) reported that the caesarean section rate was lower in women who had a successful manual rotation compared to when the fetus was unable to be rotated (2% versus 34%) 26. However, there was no control group of women for whom a manual rotation was not performed and it is not possible to know if this was due to the procedure itself or to underlying confounders such as a smaller fetus.
In a prospective cohort study with historical controls (n=61), the local labour ward policy was changed from not performing prophylactic manual rotation to routinely performing the procedure for OP position about 'half way' into the second stage of labour 12. The operative delivery rate for fetuses in the OP position fell from 73% prior to the change in policy to 23% after the policy was implemented, but this study design is subject to a significant risk of bias.
Schaffer et al (2011) re-reported their 2006 data with a control group identified retrospectively from a database and found a 9% risk of caesarean section when manual rotation was performed compared with a 41% risk when it was not 13. However, the authors had information on the fetal position at the time of birth but not earlier in the second stage of labour when the procedure was performed. Thus OP fetuses that were destined to rotate naturally to the OA position would have been included in the intervention group, but not the control group which would result in an overestimation of the caesarean section rate in the control group and of the efficacy of manual rotation.
Thus preliminary studies suggest that manual rotation reduces the risk of operative delivery but are susceptible to significant bias. A RCT would best provide unbiased answers regarding the effects of manual rotation of the fetal occiput on maternal and perinatal outcomes.
The safety of the intervention
Manual rotation has long been considered to be safe 9. One retrospective cohort study reported lower rates of complications when it was performed for OP position compared to when it was not (Table 1) 13:-
TABLE 1: Complications of manual rotation versus expectant management (Shaffer 2011)13.
|
Complication |
Manual rotation (n = 731) |
Expectant (n = 2,527) |
p |
|
Postpartum haemorrhage |
22.3% |
33.1% |
<0.001 |
|
3rd and 4th degree tears |
15.7% |
20.1% |
0.017 |
|
Cervical laceration |
2.2% |
1.0% |
0.024 |
|
Chorioamnionitis |
8.6% |
14.4% |
<0.001 |
|
Endometritis |
3.6% |
7.2% |
<0.001 |
|
5 min Apgars <7 |
1.8% |
3.7% |
0.011 |
|
Umbilical cord arterial pH <7 Base excess < -12 |
0.6% 3.5% |
1.4% 3.2% |
0.15 0.73 |
|
Shoulder dystocia |
2.1% |
1.1% |
0.064 |
|
Birth trauma |
1.09% |
1.23% |
0.77 |
Thus third and fourth degree tears, chorioamnionitis, post-partum haemorrhage, endometritis and 5 minute Apgars less than 7 all improved when prophylactic manual rotation was performed but cervical laceration was increased.
In the POP-OUT trial manual rotation will be performed at full dilatation which theoretically will minimize the risk of cervical laceration.
There is also a single case report of an umbilical cord prolapse associated with a manual rotation 27. In this report, an emergency caesarean section was performed and the baby was born alive and presumably well. Other risk factors such as amniotomy, application of a fetal scalp electrode and external cephalic version were more frequently associated with umbilical cord prolapse 27.
The timing of the intervention
Manual rotation from the OP position may be performed at full cervical dilatation or late in the first stage of labour. In a French case control study (n=147) in a labour ward where prophylactic manual rotation was performed routinely, two risk factors for inability to rotate the fetus were identified: (1) attempted rotation before full dilatation and (2) failure to progress in labour 28. Thus we consider that it would be reasonable to attempt prophylactic manual rotation after full dilatation is achieved, but relatively early in the second stage of labour, before the fetal head becomes impacted in the maternal pelvis.
Rationale for operative delivery as the primary outcome
Operative delivery was selected as the primary outcome for the POP-OUT Trial because it is clearly associated with important short and long term outcomes for the woman and her baby 6-8;29-32. Other important obstetric parameters will be measured, but reported as secondary outcomes. Reducing the rate of operative delivery for OP position is perceived to be very important by obstetricians and midwives 10;11. In high income countries, emergency caesarean section is associated with significant maternal morbidity and a five-fold increase in maternal mortality 33.
Explanation for choice of comparator
A sham procedure was chosen as a comparator to minimize the risk of performance bias. There would be substantial scope for management to differ according to treatment allocation if it was known. For example a women could be encouraged to push more strongly if her midwife was aware that a manual rotation had been performed.
Aim:
To determine the efficacy of elective manual rotation in the management of OP position in the second stage of labour.
Hypothesis:
Among women who are at least 37 weeks gestation whose baby is in the OP position early in the second stage of labour, manual rotation compared with a “sham” rotation will result in a reduction in operative delivery.
Primary objectives are to determine differences between intervention and control groups in:
Operative delivery rate (defined as vacuum, forceps and/or caesarean section deliveries)
Secondary objectives are to determine differences between intervention and control groups in:
Caesarean section
Combined measure of serious maternal morbidity and mortality within six weeks of birth.
Combined measure of serious perinatal/neonatal morbidity and mortality within six weeks of birth.
Trial design
The POP-OUT trial is designed as a superiority, double-blinded, multi-centred, randomised controlled clinical trial with two parallel groups and a primary endpoint of operative delivery. Randomization will be performed as block randomization with a 1:1 allocation.
Study settings: Hospitals in Australia which have 2,000 or more deliveries per year:
Canterbury Hospital, NSW
The John Hunter Hospital, NSW
The Nepean Hospital, NSW
The Royal Hospital for women, Randwick, NSW
The Royal Prince Alfred Hospital, NSW
The Women and Children's Hospital, SA
We do not intend to recruit in any other centres. A list of participating centres may be found at:
www.popout.me/participating-hospitals
Eligibility criteria
Inclusion criteria:
age ≥ 18 years
singleton pregnancy
≥ 37 weeks of gestation
planned vaginal birth
cephalic presentation
full cervical dilatation
occiput posterior position confirmed by ultrasound where the occiput is < 45o from the midline.
Exclusion criteria:
Most exclusion criteria were selected on the basis of predisposition to requiring an operative delivery:-
clinical suspicion of cephalopelvic disproportion
previous caesarean section
brow or face presentation
'Pathologic' CTG according to RCOG classification plus either baseline >160 beats per minute or reduced variability
fetal scalp pH < 7.25 or lactate > 4
known or suspected chorioamnionitis
intrapartum haemorrhage > 50mL
temperature ≥ 38.0oC in labour
pre-existing maternal diabetes
suspected fetal bleeding disorder (theoretical risks associated with procedures involving manipulation of fetal position)
known major anatomical fetal abnormality (could influence safety or efficacy of manual rotation)
Eligibility criteria for study centres:
Ability to provide a 24 hour on-call service using experienced operators to perform the intervention.
Individuals who will perform the intervention:
Obstetricians or midwives who are experienced in performing a manual rotation and have performed at least 20 procedures. All operators will complete a questionnaire outlining their technique and experience.
Intervention: Manual rotation
Intervention description: Manual rotation is performed at full dilatation if the fetal position is OP. The technique employed will be at the discretion of the operator performing the procedure.
With the membranes ruptured, a vaginal examination is performed and the woman is asked to bear down. Constant pressure is exerted with the index finger against the lambdoid suture to rotate fetal head. This may take 2 to 3 contractions and the position is commonly held for two contractions while the woman bears down to reduce the risk of reverting back to the OP position.
Alternatively, the examiner places two fingers behind the fetal ear or the entire hand behind the occiput and applies constant flexion and rotation to the fetal head.
For purposes of the POP-OUT Trial, the procedure will be described as a 'digital rotation' if only the fingers are used and as a 'manual rotation' if the whole hand is used.
Comparator: Sham procedure
Comparator description:
Women randomized to the “sham rotation” will have the same apparent vaginal examination as the intervention but no rotational force will be applied. The woman is asked to bear down. The accoucheur places fingers in the vagina over 5 contractions as if s/he were performing a manual rotation.
The timing of the intervention:
Once full dilatation has been diagnosed: When the woman has the first urge to push or after one hour, whichever occurs first.
Criteria for discontinuing or modifying the intervention:
The intervention or sham will be discontinued if there is a clinical necessity or at the request of the participant. This could occur if there is evidence of fetal compromise necessitating emergent delivery or if the participant is in significant discomfort.
Each operator will complete a data collection form at the time of the procedure or sham, which will describe in detail what was done. Adherence with treatment allocation will be monitored by comparing these datasheets with the computer randomisation records.
All interventions and usual care provided by doctors and midwives looking after the participant will be allowed. However, if the doctor is intending to perform an operative delivery or a manual rotation, the woman will not be randomised. Data will be collected about use and timing of any manual rotations performed by the participant's carers.
Outcomes
Primary Outcome:
Operative delivery (vacuum, forceps and/or caesarean section).
Secondary Outcomes:
Caesarean section (reported as proportion of participants who had a caesarean section)
Serious maternal morbidity or mortality (combined outcome):-
This will include one or more of the following: post-partum haemorrhage requiring blood transfusion, third or fourth degree perineal trauma; dilatation and curettage for bleeding or retained placental tissue; cervical laceration; vertical uterine incision; vulvar or perineal haematoma; pneumonia; venous thromboembolism requiring anticoagulation; wound infection requiring hospital stay more than 7 days; readmission to hospital for obstetric related causes; wound dehiscence; maternal fever of at least 38.5oC on two occasions at least 24 hours apart not including the first 24 hours; bladder, ureter or bowel injury requiring repair; genital-tract fistula; bowel obstruction; admission to intensive care unit. This will be reported as a proportion of participants with serious morbidity or mortality.
Serious perinatal/neonatal morbidity or mortality within 6 weeks of birth (combined outcome):-
This will include one or more of the following: shoulder dystocia requiring manouvres other than McRoberts/suprapubic pressure or resulting in neonatal injury, 5 minute Apgars < 4; arterial cord pH < 7.0 or lactate > 10 or base excess < -15; serious birth trauma, seizures < 24 hours of age, intubation/ventilation > 24 hours, tube feeding > 4 days, admission to neonatal intensive care > 4 days, neonatal jaundice requiring phototherapy. This will be reported as a proportion of participants with serious morbidity or mortality.
Other Outcomes:
During delivery admission:
length of second stage (median)
time from intervention or sham until delivery (median)
estimated blood loss at delivery (median: visual estimation by midwife or doctor)
any perineal/vaginal trauma requiring suturing (proportion)
length of hospital stay (median)
At 6 weeks:
still breast feeding (proportion)
satisfaction with birth (VAS scale) (median)
saw a health professional for depression since delivery (proportion)
health related quality of life (SF-12) (median)
At 6 months:
still breast feeding (proportion)
saw a health professional for depression since delivery (proportion)
health related quality of life (SF-12) (median)
At one year:
still breast feeding (proportion)
saw a health professional for depression since delivery (proportion)
health related quality of life (SF-12) (median)
pelvic floor function (bowel, urinary, prolapse, and sexual function domains - using the Australian pelvic floor function questionnaire 34 (medians)
Figure 1 provides an overview of the conduct of the POP-OUT Trial.
Figure 1: The POP-OUT Trial Schema
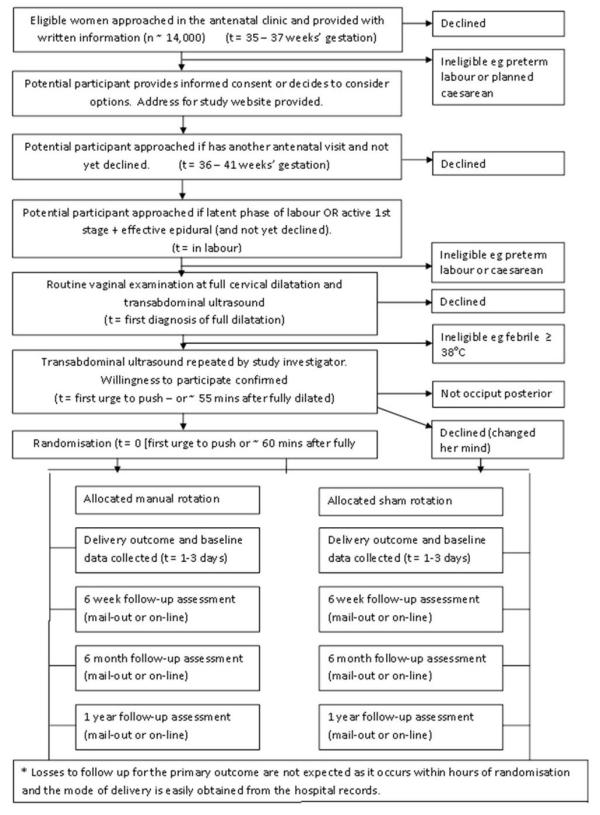
Sample Size: 254
The sample size was calculated on the basis of the primary outcome. The power calculation was based on our prospective cohort study of 160 women which was completed in May 2009 3 which showed an operative delivery rate of 68% in the OP group; as well as our survey of obstetricians conducted in 2010 who indicated they would perform a manual rotation for OP position if it reduced the rate of operative delivery from 68% to 50% 10. To detect a reduction in the rate of operative delivery from 68% in the control group to 50% in the intervention group, a sample size of 127 women in each group (total=254) will be required to have 80% power of finding a result. Alpha = 0.05 (2-tailed), Beta = 0.20 (Epi-Info version 3.3.2).
TABLE 2: The POP-OUT study time-line for the schedule of enrolment, allocation and follow-up
|
|
Enrolment |
Allocation |
Post-allocation |
|||||
|
Timepoint |
35-37 wks gestation |
1st stage labour |
During 2nd stage of labour |
Immediately after allocation |
1-3 days |
6 wks |
6 mths |
12 mths |
|
1st Eligibility screen |
X |
|
|
|
|
|
|
|
|
2nd eligibility screen |
|
X |
|
|
|
|
|
|
|
Informed consent |
X |
X |
|
|
|
|
|
|
|
Allocation |
|
|
X |
|
|
|
|
|
|
Intervention |
|
|
|
X |
|
|
|
|
|
Assessments: |
||||||||
|
Labour & Delivery |
|
|
X |
X |
X |
|
|
|
|
Operative delivery |
|
|
|
|
X |
|
|
|
|
Perineal outcome |
|
|
|
|
X |
|
|
|
|
Blood loss |
|
|
|
|
X |
|
|
|
|
Maternal complications |
|
|
|
|
X |
X |
|
|
|
Hospital stay |
|
|
|
|
X |
|
|
|
|
Readmission |
|
|
|
|
|
X |
|
|
|
Neonatal outcomes |
|
|
|
|
X |
X |
|
|
|
NICU admission(s) |
|
|
|
|
X |
X |
|
|
|
Satisfaction with birth |
|
|
|
|
|
X |
|
|
|
Breast feeding |
|
|
|
|
|
X |
X |
X |
|
Health related quality of life |
|
|
|
|
|
X |
X |
X |
|
Pelvic floor |
|
|
|
|
|
X |
X |
X |
|
Depression |
|
|
|
|
|
X |
X |
X |
Randomization/allocation concealment
Randomization will be stratified by parity, hospital site and epidural due to the potentially strong association between operative delivery (the primary outcome) and each of these factors. Randomization will be centrally controlled using computerized sequence generation which can be accessed 24 hour per day using a toll-free telephone line.
In order to reduce the risk of randomising an ineligible participant, randomisation will occur immediately before the intervention or sham procedure is to be performed. An example of a participant becoming ineligible would be if the fetus rotated from occiput posterior to occiput transverse position. Each investigator will complete a data collection form at the time the manual rotation or sham procedure is performed outlining the treatment allocation, clinical findings, and whether or not the fetus was successfully rotated.
Blinding
The following groups will be masked:
The participants
The clinicians caring for the participant (including doctors and midwives)
The data collectors
The statisticians who will perform the analysis
Unblinding
Unblinding will occur if the clinician requests it on the basis of clinical need or if the participant insists.
Data collection, management and analysis
Study conduct:
Consent will occur at three possible timepoints:
Antenatally,
In the latent phase of labour
In the active phase of the first stage of labour, with an effective epidural anaesthesia
An ultrasound will be performed at full dilatation by the clinician caring for the woman and the findings will be recorded on a data sheet immediately afterwards.
An hour after full dilatation or at the first urge to push a study investigator (with no clinical responsibility for the woman in the trial) will confirm the OP position by a second (pre-procedure) bed-side ultrasound. If the fetal position is still OP and the woman still wishes to participate then the study investigator will randomise the woman to either manual or sham rotation. The treatment allocation will be recorded on a randomisation sheet which the investigator will keep on their person and not show to any of the participant's carers.
After the manual rotation or sham has been performed the ultrasound will be repeated, ensuring that the woman and her carers do not see the screen. The investigator will leave and the woman will have her usual care from this point onwards. The investigator will record the findings on vaginal examination, details of the procedure and post-procedure ultrasound findings on the same data sheet as the pre-procedure ultrasound. The study investigator will also keep this data sheet on their person and not show it to any of the participants' carers.
Primary Outcome
Mode of delivery will be ascertained from the medical records.
Other outcomes
Labour and delivery outcomes, perineal trauma, blood loss, duration of hospitalisation, short term neonatal outcomes, and admission to the neonatal intensive care unit, maternal or neonatal readmission to the same institution, and other components of the combined secondary outcomes will be ascertained by a study investigator not involved in clinical care, using the medical records and by contacting the participants' clinician for further information if required. Maternal depression, health related quality of life (SF-12), birth satisfaction (VAS), maternal or neonatal readmission to another institution, ongoing breast feeding, pelvic floor symptoms and components of the combined secondary outcomes will be collected by structured maternal questionnaires at 6 weeks, 6 months and 12 months post-delivery as outlined in section 15. Questionnaires will be completed by mail-out, on-line via the trial website and by telephone depending on the participants' preferences. Data collectors will be unaware of the treatment allocation at all times.
As the primary outcome is mode of delivery and randomisation occurs during the second stage of labour, we expect 100% ascertainment for the primary outcome.
Study investigators will perform site visits about four times per year to promote recruitment, provide education for clinical staff and site investigators and audit of centre medical records to verify accuracy of data collected by sites.
Participants will receive a phone call at each time point by research staff not involved in her care to ask her preference for follow-up. Unless she declines further participation, each participant will receive a reminder phone call and will be offered completion of the questionnaire by telephone if they feel they cannot complete it by mail or on-line.
Data management
Data collected will be entered into a registered electronic database by research staff blinded to treatment allocation who are not involved in the clinical care of participants. Hardcopies of participants' data will be stored in a locked office. The electronic database will include the study identification number but no directly identifying data such as medical record number, date of birth or personal address. The de-identified database will be backed up on a server at Royal Prince Alfred Hospital. Data linking identifying details to the study number will be kept at a separate location in a locked filing cabinet. At the end of the study, data will be kept in a locked filing cabinet, and de-identified electronic data will be kept on a portable medium such as a USB drive in a separate secure location at Royal Prince Alfred Hospital.
All electronic data will be checked for accuracy by a second member of the research team and any apparent data entry errors will be discussed by the primary investigators and investigated/corrected as required.
Statistical methods
Analysis:
Analysis will be by intention to treat (according to treatment allocation), including withdrawals and losses to follow up. Losses to follow up for the primary outcome are not expected because randomisation will occur at full dilatation and the primary outcome is the mode of delivery.
The results will be reported according to CONSORT guidelines.
Demographics and other potential confounders will be compared by treatment allocation in a univariate analysis. Categorical outcome measures will be compared by proportions (chi-squared test), means for normally distributed data (t-test), or rank order for non-normally distributed data (Mann-Whitney-U test).
A logistic regression analysis of treatment allocation and other variables on the primary outcome measure, operative delivery, will be performed. The following variables will be considered for the logistic regression model: maternal body mass index, maternal age, maternal height, maternal ethnicity, gestation, induction of labour, gestational diabetes, neonatal gender, and RCOG CTG classification in the second stage of labour. Parity, study site and the presence of epidural for intrapartum analgesia at the time of randomisation will not be included because randomisation is stratified for these variables. Only variables where p < 0.25 in the univariate regression will be included in the multivariate model. Continuous variables which do not show a linear association with the logit function will be divided into quartiles and treated as categorical. Interaction terms will be considered for treatment allocation versus each of the other variables and where clinically appropriate between non-treatment variables. p < 0.01 will be considered evidence of interaction. Terms will be excluded from the model in a stepwise backward manner until all remaining terms are both statistically significant (p < 0.05) and clinically significant (that is, removal of the term results in a clinically significant change in the estimate of the odds ratio of treatment allocation for the primary outcome). The analysis will be performed using SAS 9.2 (or a more recent version of SAS).
Additional analyses:
Subgroup analyses will be performed according to the technique of manual rotation employed (manual/whole hand versus digital/fingers) and according to operator ability (data will be divided into two approximately equal groups according to the success rate of the operator who performed the manual rotation).
Data safety Monitoring Committee
Draft terms of reference for a data and safety monitoring committee provide for potential cessation of the trial if significant safety concerns are raised. The data and safety monitoring committee will consist of 3 people who are not involved in the study and do not have a working relationship with the primary investigators. Adverse events will be reported to the committee.
Interim analysis and stopping rules
There will be no interim analysis. The Data Safety Monitoring Committee may advise that the trial be stopped if significant concerns about the safety of manual rotation are found.
Harm
Any serious complications will be referred to the Data Monitoring Committee.
Auditing
There will be no external auditing of the trial.
Ethics and dissemination
Research Ethics Approval
This study has been approved by the Ethics Review Committee (RPAH Zone) of the Sydney Local Health District, Sydney, Australia, Protocol number X110410.
Protocol Amendments
If modification to the study protocol is considered necessary then permission will be sought from the ethics committee and the changes will be described in the final report.
Consent
Participants will be provided with written information via information pamphlets, posters and the trial website. Informed consent will be obtained by research midwives or midwives/medical staff involved in potential participants' care. A detailed information sheet will be provided to all participants. Participants will be informed of the potential risks of manual rotation, including umbilical cord prolapse, given the opportunity to ask questions and informed that they have the right to change their mind at any time.
Confidentiality
All the information collected from the study will be treated confidentially, and only the researchers will have access to it. Hard copies of data collection forms will be stored in a locked office. The electronic database will be de-identified and stored at a different location to codes linking identifying data to study identification numbers. The electronic database will be on Microsoft Access, password-protected, and only accessible by research staff.
Declaration of interests
Ms Hala Phipps is financially supported by a research grant for the POP-OUT Trial from the NHMRC.
Ms Hala Phipps, Dr Brad de Vries and Professor Jon Hyett are co-authors on a Cochrane systematic review assessing the efficacy of prophylactic manual rotation for reducing operative delivery which is likely to include the results of this trial when they become available.
Access to data
All principal investigators will have access to the de-identified data set. If ethics permission is obtained, it will be possible to re-identify the data for purposes of future research. The de-identified dataset will be available to the principal investigators on an ongoing basis. Site investigators will have access to their own site's data and may be provided with the complete de-identified dataset on request.
Ancillary and post-trial care
All participants will have access to hospital care with a specialist obstetrician if any complications occur as a result of the trial. For example, in the event of a umbilical cord prolapse leading to an emergency caesarean section, the participant will have the opportunity to see a specialist obstetrician during her hospital stay and for follow-up visits. The obstetrician will be able to refer the woman to other appropriate services such as clinical psychologists.
Dissemination policy
The results of the trial may be presented at one or more medical conferences. The study will be submitted to peer review journals for consideration for publication. The investigators will undertake to provide data to authors of appropriate systematic reviews such as those contained in the Cochrane database of systematic reviews. Individual participants will be able to contact the study investigators via details on the information sheet. They will also be able to contact the primary investigators for a period of at least three years after all follow-up is completed via the 'contact' tab on the trial website (www.popout.me).
Authorship eligibility guidelines and any intended use of professional writers
|
The first draft of the trial report will be made by the primary investigators and other investigators will have the opportunity to participate in subsequent versions. We do not intend to employ professional writers for this purpose. |
|
Plans, if any, for granting public access to the full protocol, participant-level dataset, and statistical code If the ethics committee permits it, a de-identified dataset will be made available to researchers on request. SAS code will be made available to anyone who asks.
|
Publication plan:
Start date: 16th April 2012
Finishing recruitment: December 2014
Finishing follow-up: December 2015
Reporting date: December 2016
Contributorship statement
Hala Phipps: Primary investigator, trial design, applications for funding, overall co-ordination of the trial, data management, drafting the first version of the report.
Jon Hyett: Trial supervision, trial design, applications for funding, contribution to ongoing conduct of the trial and contribution to writing the report.
Sabrina Kuah: Applications for funding, site co-ordination, performing the intervention, contribution to ongoing conduct of the trial and contribution to writing the report.
John Pardey: Applications for funding, site co-ordination, performing the intervention, contribution to ongoing conduct of the trial and contribution to writing the report.
Joanne Ludlow: Applications for funding, site co-ordination, performing the intervention, contribution to ongoing conduct of the trial and contribution to writing the report.
Andrew Bisits: Applications for funding, site co-ordination, performing the intervention, contribution to ongoing conduct of the trial and contribution to writing the report.
Felicity Park: Applications for funding, site co-ordination, performing the intervention, contribution to ongoing conduct of the trial and contribution to writing the report.
David Kowalski: Site co-ordination, performing the intervention, contribution to ongoing conduct of the trial and contribution to writing the report.
Bradley de Vries: Trial concept and supervision, trial design, applications for funding, overall co-ordination of the trial, performing the intervention, data management, drafting the first version of the report.
Competing interests
The authors have stated explicitly that there are no conflicts of interest.
Funding
National Health and Medical Research Council (project grant ID 1029664)
Luke Proposch Perinatal Research Scholarship: Royal Australian and New Zealand College of Obstetrics and Gynaecology Research Foundation
Reference List
(1) Macara LM, Murphy KW. The contribution of dystocia to the cesarean section rate. Am J Obstet Gynecol 1994; 171:71-77.
(2) Senecal J, Xiong X, Fraser WE. Effect of fetal position on second-stage duration and labor outcome. 2005; 105:763-772.
(3) Carseldine WJ, Phipps H, Zawada SF, Campbell NT, Ludlow JP, Krishnan SY et al. Does occiput posterior position in the second stage of labour increase the operative delivery rate? Aust N Z J Obstet Gynaecol 2013; 53(3):265-270.
(4) Hall MH, Bewley S. Maternal mortality and mode of delivery. Lancet 1999; 354(9180):776.
(5) BARROS FC, Victoria CG, Barros AJ, Santos IS, Albernaz E, Matijasevich A et al. The challenge of reducing neonatal mortality in middle-income countries: findings from three Brazilian birth cohorts in 1982, 1993, and 2004. The Lancet 2005; 365:847-854.
(6) Bahl R, Strachan B, Murphy DJ. Outcome of subsequent pregnancy three years after previous operative delivery in the second stage of labour: cohort study. 2004; 328:311.
(7) Islam A, Hanif Khan A, Murtaza Nosheen J. Vacuum extraction and forceps deliveries; comparison of maternal and neonatal complications. 2008; 15:87-90.
(8) Murphy DJ, Macleod M, Bahl R, Strachan B. A cohort study of maternal and neonatal morbidity in relation to use of sequential instruments at operative vaginal delivery. 2011; 156:41-45.
(9) SOGC. Guidelines for operative vaginal birth. Number 148, May 2004. Int J Gynaecol Obstet 2004; 88:229-236.
(10) Phipps H, de Vries B, Lee PN, Hyett JA. Management of occiput posterior position in the second stage of labour: a survey of obstetric practice in Australia and New Zealand. 2012; 52:450-454.
(11) Phipps H, de Vries B, Jagadish U, Hyett J. Management of occiput posterior position in the second stage of labour: A survey of midwifery practice in Australia. Birth [In Press]. 2014.
Ref Type: Abstract
(12) Reichman O, Gdansky E, Latinsky B, Labi S, Samueloff A. Digital rotation from occipito-posterior to occipito-anterior decreases the need for cesarean section. Eur J Obstet Gynecol Reprod Biol 2008; 136:25-28.
(13) Shaffer BL, Cheng YW, Vargas E. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. 2011; 24:65-72.
(14) Simkin P. The fetal occiput posterior position: state of the science and a new perspective. 2010; 37:61-71.
(15) Gardberg M, Laakkonen E, Salevaara M. Intrapartum sonography and persistent occiput posterior position: a study of 408 deliveries. 1998; 91:746-749.
(16) Sizer AR, Nirmal DM. Occipitoposterior position: associated factors and obstetric outcome in nulliparas. 2000; 96:749-752.
(17) Lieberman E, Davidson K, Lee-Parritz A, Shearer E. Changes in fetal position during labor and their association with epidural analgesia. 2005; 105:974-982.
(18) Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: A retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med 2006; 19:563-568.
(19) Fitzpatrick M, McQuillan K, O'Herlihy C. Influence of persistent occiput posterior position on delivery outcome. Obstet Gynecol 2001; 98:1027-1031.
(20) Ponkey SE, Cohen AP, Heffner LJ, Lieberman E. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol 2003; 101:915-920.
(21) Hanson L. Second-stage labor care: challenges in spontaneous bearing down. 2009; 23:31.
(22) Souka AP, Haritos T, Basayiannis K, Noikokyri N, Antsaklis A. Intrapartum ultrasound for the examination of the fetal head position in normal and obstructed labor. 2003; 13:59-63.
(23) Benavides L, Wu JM, Hundley AF. The impact of occiput posterior fetal head position on the risk of anal sphincter injury in forceps-assisted vaginal deliveries. 2005; 192:1702-1706.
(24) Cheng YW, Hubbard A, Caughey AB, Tager IB. The Association Between Persistent Fetal Occiput Posterior Position and Perinatal Outcomes: An Example of Propensity Score and Covariate Distance Matching. 2010; 171:656-663.
(25) Abrahams I. Guidelines for operative vaginal birth. J Obstet Gynaecol Can 2004; 26:965.
(26) Shaffer BL, Cheng YW, Vargas JE, Laros RK, Jr., Caughey AB. Manual rotation of the fetal occiput: predictors of success and delivery. Am J Obstet Gynecol 2006; 194:e7-e9.
(27) Usta IM, Mercer BM, Sibai BM. Current obstetrical practice and umbilical cord prolapse. 1999; 16:479-484.
(28) Le Ray C. Manual rotation in occiput posterior or transverse positions: risk factors and consequences on the cesarean delivery rate. Obstet Gynecol 2007; 110:873-879.
(29) Jackson N, Paterson-Brown S. Physical sequelae of caesarean section. [Review] [65 refs]. 2001; 15:49-61.
(30) Zelop C, Heffner LJ. The downside of cesarean delivery: short-and long-term complications. 2004; 47:386.
(31) Guise JM, McDonagh MS, Osterweil P, Nygren P, Chan BK, Helfand M. Systematic review of the incidence and consequences of uterine rupture in women with previous caesarean section. [Review] [30 refs]. 2004; 329:19-25.
(32) Sarsam SE, Elliott JP, Lam GK. Management of wound complications from cesarean delivery. 2005; 60:462-473.
(33) Lumbiganon P, Laopaiboon M, Gulmezoglu AM, Souza JP, Taneepanichskul S, Ruyan P et al. Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007-08.[Erratum appears in Lancet. 2010 Dec 4;376(9756):1902]. 2010; 375:490-499.
(34) Baessler K, O'Neill SM, Maher CF, Battistutta D. A validated self-administered female pelvic floor questionnaire. Int Urogynecol J 2010; 21(2):163-172.

